Our purpose
The BMJ Technology Assessment Group (BMJ-TAG) plays a crucial role in supporting NICE (National Institute for Health and Care Excellence) by conducting independent assessments of new health technologies. Our purpose is to provide rigorous evaluations of clinical and cost-effectiveness evidence, helping NICE make informed decisions about whether treatments should be recommended for use in the NHS (National Health Service) in England.
BMJ-TAG is one of 11 centres of excellence funded by the NIHR (National Institute for Health Research) to evaluate evidence submitted by companies during the health technology assessment (HTA) process. In the NICE HTA pathway, BMJ-TAG critically appraises the clinical and economic evidence, conducts its own analyses, and produces an evidence assessment report. This report informs the NICE Evaluation Committee, helping guide their recommendations on whether a treatment should be made available on the NHS.
The objectives of BMJ-TAG are therefore to independently assess the clinical and cost-effectiveness of health technologies, support NICE in making evidence-based recommendations, and ensure that scarce NHS resources are used efficiently.
Our work helps enable timely patient access to effective treatments while maintaining high methodological rigour and impartiality standards.


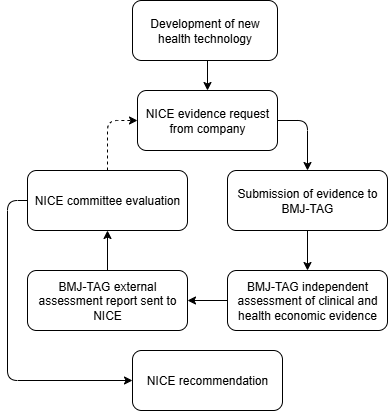
Our process
BMJ-TAG supports NICE through the independent assessment of clinical and health economic evidence, helping to inform NICE recommendations.
The evidence passed to BMJ-TAG is requested by NICE, and is used to inform external assessment report produced by BMJ-TAG, which in turn is used to inform the evaluation by NICE committees.
If robust clinical and cost-effectiveness evidence is provided that demonstrates a new technology is cost-effective, NICE may recommend it for routine use within NHS England.
If the decision of cost-effectiveness is highly uncertain, NICE may request additional evidence from the company which is passed to BMJ-TAG. The process continues until sufficient evidence is available for the NICE committee to make a robust decision on the cost-effectiveness of the health technology.
Our focus
Medicines appraisal process
Device appraisal process
Commissioned critical appraisals
Our work
Our group provides independent, evidence based evaluations of healthcare technologies to support national decision making in England.
We carry out assessments for medicines, medical devices, diagnostic tests, and other healthcare interventions to ensure patients receive safe, effective and cost-efficient care.
While initially expensive, these drugs may lead to long-term savings by preventing hospitalisations and managing complications. BMJ-TAG’s analysis would consider these long-term effects. Therefore, David Ramsden, the Chief Executive of Cystic Fibrosis Trust, has hailed this decision as a significant achievement for individuals with CF, drastically improving their quality of life and life expectancy. This ensures secure, continued treatment access for thousands of people affected by CF in the UK.
BMJ-TAG team’s research informs negotiations between the NHS, NICE, and pharmaceutical companies, often leading to reduced drug prices and improved patient access. This evidence-based work ensures that life-extending and quality-of-life-improving treatments are accessible and cost-effective for the NHS, ultimately benefitting many patients.
- Supporting informed decisions based on evidence
- Improving patient lives with effective treatments
- Ensuring value for money through careful analysis

Cystic fibrosis medicines appraisal
Cystic fibrosis (CF) is a life-limiting genetic condition affecting over 9000 individuals in England. Characterised by a progressive decline in lung function and frequent, severe respiratory infections, CF historically has had a significant impact on life expectancy, with an average age of death of just 36 years. Recently, a new generation of treatments has emerged that target the underlying cause of CF. Still, they were only available in clinical trials, and none were routinely available on the NHS.
BMJ-TAG examined the costs and benefits of three such treatments (KaftrioⓇ, SymkeviⓇ and OrkambiⓇ). Their research concluded that they potentially offer life-changing patient benefits, but at an exceptionally high price for the NHS. After discussions between NICE, NHS, and Vertex Pharmaceuticals (manufacturer of the drugs), informed by further research by BMJ-TAG, all three drugs were recommended for use.
The primary goal of healthcare is to improve patient outcomes. If a treatment offers significant, life-changing benefits, even at a high cost, it can be deemed worthwhile. Cystic fibrosis drugs drastically improve life expectancy and quality of life, which is a substantial benefit to patients.

At BMJ-TAG, we are committed to transparency and integrity in all aspects of our work. As an independent academic group, we are not funded by pharmaceutical companies or any of the manufacturers whose technologies we assess. All of the department is required to declare any conflicts of interest, and we ensure that no one with a potential conflict is involved in the assessment of a related technology. We recognise our responsibility to be open about the research we conduct, the rigour of our methods, and any limitations of the evidence we review, so that our advice remains credible, impartial and trusted by all stakeholders.
“We are committed to transparency in our research, methods, and evidence assessment, so that our advice is not only fair and robust but is seen as such by all stakeholders.”
Dr Steve Edwards
Director of Health Technology Assessment, BMJ-TAG
Our values
Our values give us the focus and direction to bring about our vision for a healthier world by sharing knowledge and expertise to help health professionals improve healthcare outcomes.












