Our purpose
The BMJ Technology Assessment Group (BMJ-TAG) supports NICE (National Institute for Health and Care Excellence) by delivering independent evaluations of new health technologies. We assess clinical and cost-effectiveness evidence to help NICE decide which treatments should be recommended for use in the NHS (National Health Service).
Funded by NIHR (National Institute for Health Research), BMJ-TAG is one of the 11 centres of excellence for health technology assessment (HTA). We critically appraise company-submitted evidence, conduct our own analyses, and produce reports that guide the NICE Evaluation Committees.
We aim to ensure that NHS resources are used efficiently and patients gain timely access to effective treatments. Our work is grounded in methodological rigour, independence and a commitment to improving healthcare outcomes.
Our process
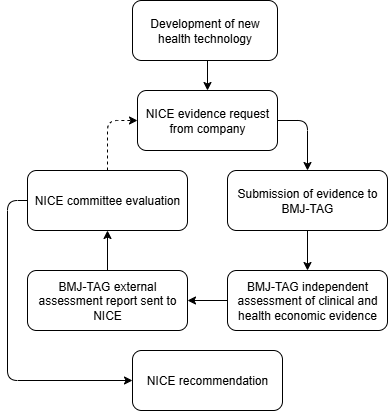
BMJ-TAG supports NICE by independently assessing clinical and health economic evidence to inform recommendations on new health technologies.
NIHR commissions BMJ-TAG to evaluate evidence submitted by companies. We produce external assessment reports, which inform NICE committee discussions and decision-making.
If robust evidence shows a new treatment is clinically and cost-effective, NICE may recommend it for routine use within NHS England. When evidence is uncertain, additional evidence may be requested from the company until a robust decision can be made.
Our focus
Medicines appraisal process
HealthTech appraisal process
Commissioned critical appraisals
Our work
Our group provides independent, evidence-based evaluations of healthcare technologies to support national decision making in England.
Our cystic fibrosis medicines appraisal
Cystic fibrosis (CF) is a life-limiting genetic condition affecting over 9000 individuals in England. Characterised by a progressive decline in lung function and frequent, severe respiratory infections, CF historically has had a significant impact on life expectancy, with an average age of death of just 36 years. Recently, a new generation of treatments has emerged that target the underlying cause of CF. Still, they were only available in clinical trials, and none were routinely available on the NHS.
BMJ-TAG examined the costs and benefits of three such treatments (KaftrioⓇ, SymkeviⓇ and OrkambiⓇ). Their research concluded that they potentially offer life-changing patient benefits, but at an exceptionally high price for the NHS.
After discussions between NICE, NHS, and Vertex Pharmaceuticals (manufacturer of the drugs), informed by further research by BMJ-TAG, which helped NICE negotiate for lower treatment prices, all three drugs were recommended for use.
“This is a fantastic moment for many people with cystic fibrosis and their families – ending uncertainty and helping to ensure that everyone who can benefit can access these vital medicines – now and in the future.”
David Ramsden
Chief executive of the Cystic Fibrosis Trust

“As an academic group, we are not funded by pharmaceutical companies or the manufacturers of the technologies we assess. All department members declare any potential conflicts of interest, and no one with a conflict is involved in a related assessment. We are open about our methods, the evidence we review and any limitations, ensuring our advice remains rigorous, impartial and trusted by stakeholders.”
Dr Steve Edwards
Director of Health Technology Assessment, BMJ-TAG
Our values
Our values give us the focus and direction to bring about our vision for a healthier world by sharing knowledge and expertise to help health professionals improve healthcare outcomes.